- Perspective – July 2022
- Advert : Columbus Stainless
- Industry Analysis
- Advert & Advertorial – EMV Africa
- Member Benefits
- Member Innovation
- Market Intelligence
- Advert – Beyond Stainless Steel Workshop
- Professional Profile
- Advertorial – Innov-X-Africa
- Advert – Innov-X-Africa
- Market Analysis
- Technical Analysis
- Technical Insight
- Advert – Unique Welding
- Africa Market Intelligence
- Member Events
NICKEL-CONTAINING STAINLESS STEELS AND POTENTIAL ALTERNATIVE GRADES
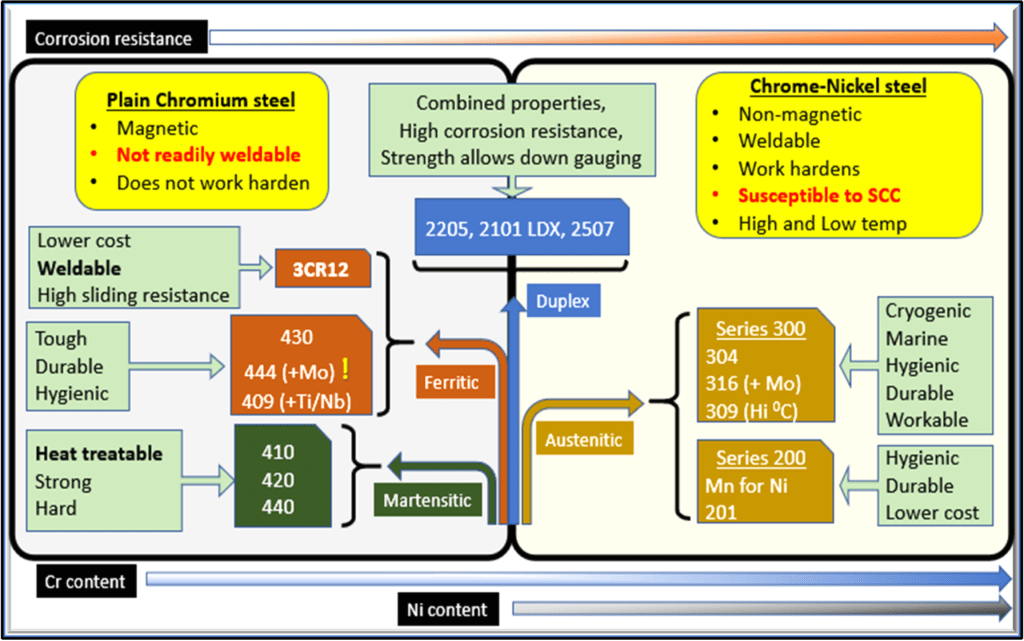
 Stainless steel isn’t a single material, but a family consisting of five ‘clans’, each of which is home to many grades. These five types of stainless steel - ferritic, austenitic, martensitic, duplex and precipitation hardening - are defined by their individual grain crystal structure.
Stainless steel isn’t a single material, but a family consisting of five ‘clans’, each of which is home to many grades. These five types of stainless steel - ferritic, austenitic, martensitic, duplex and precipitation hardening - are defined by their individual grain crystal structure.
Nickel is an important alloying addition in more than two-thirds of the stainless steel produced today. This includes the austenitic, precipitation hardening and duplex grades that are dependent on nickel for its properties. Lesser amounts of nickel are also found in some of the ferritic and martensitic grades.
Another important element is chrome which is the key alloy that makes stainless steels ‘stainless’. When more than 10.5 % is added to steel, a protective passive oxide film forms on the surface to make the material corrosion resistant with a bright appearance. In general, the more chrome added, the higher the corrosion resistance.
Some of the early stainless steels also contained nickel, resulting in improved properties, and nickel-containing grades have been in use ever since.
 Ferritic Structure |
 Austenitic Structure |
However, nickel may be seen as a relatively high-cost alloying addition, due to the volatility in its price. It would therefore be useful to revisit the reasons why nickel is added to stainless steel and to what extent the influence of nickel is needed in specific applications.
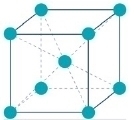
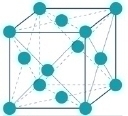
The primary function of nickel is to stabilise the austenitic crystal structure. Nickel changes the ferritic crystal structure of stainless steel to an austenitic structure (face-centred cubic). This structure is particularly tough and ductile. This property, and others, are responsible for the versatility of the various grades of nickel-containing stainless steel.
The major chemical elements making up stainless steel can be divided into two groups. The first is a group of ferrite formers including iron and chrome. The other is austenite formers such as nickel and nitrogen.
To stabilise the austenitic crystal structure, there must be a strong enough presence of austenite-forming elements in the alloying mix. The minimum amount of nickel that can stabilise the austenitic structure at room temperature is around 8%. This is the reason for the 8% nickel present in grade 304 which is the most widely used grade of stainless steel.
Grade 304 contains 18% chrome and 8% nickel and is often referred to as 18-8 stainless steel. This chemical composition was developed in Germany in the early 20th century. It was used for chemical plants and to clad the roof of the Chrysler Building in New York as one of the earliest architectural applications in the 1930s.
Manganese was first used as an addition to stainless steel in the 1930s. The 200-series of low-nickel, austenitic grades was developed further during the 1950s when nickel supply was scarce during the Korean war. These grades have an austenitic structure with a portion of their nickel content replaced by manganese.
A nickel imperative
However, not all nickel can be replaced whilst the structure remains fully austenitic. All the high-manganese austenitic stainless steel has additions of nickel to ensure enough austenite forming content. To reduce the nickel content, even more, the chemical balance is adjusted by reducing the chrome (ferrite former) content. However, this reduction of chrome negatively impacts the corrosion resistance of manganese-containing alloys compared to the standard 300-series nickel grades.
More recent improvements in melting technology at stainless steel mills have allowed the controlled addition of increased amounts of nitrogen. Nitrogen is also a potent austenite former like nickel.
As the austenite forming content in the alloy is reduced in the ways described above, the structure of the stainless steel changes from 100% austenite to a mixture of austenite and ferrite crystal structures. Grades with this deliberate reduction of austenite formers are referred to as the duplex stainless steel grades. The nickel continues to stabilise the structure of the austenite phase which would be between 50% and 60% of the total material. Commercial duplex grades, even the ‘lean duplexes’, contain at least 1% nickel. Most duplex stainless steels have a higher chrome content than the standard austenitic grades for higher corrosion protection. Chrome is a ferrite former, and, with increased chrome levels, increased levels of nickel are required to maintain the metallurgical balance. This principle also applies to manganese-containing austenitic, such as the 200 series.
Duplex strength
This dualistic or two-phase structure of the duplex grades makes it, in general, inherently stronger than the standard austenitic grades. The slightly higher chrome content also makes duplex grades more corrosion resistant than standard grades.
Reducing the nickel content further creates grades with no austenite at all. These grades have a completely ferritic structure like iron and carbon steel at ambient temperatures. It is important to note that not even ferritic stainless steel grades are completely nickel-free.
Nickel is added to lower the Ductile-To-Brittle Transition Temperature (DBTT) i.e. the temperature below which the alloy becomes brittle. Some of the highly-alloyed super-ferritic grades contain an intentional addition of nickel to improve the DBTT, especially of welds. It must be stated that the nickel levels in austenitic stainless makes it tough at cryogenic temperatures with no DBTT, i.e. it will not exhibit brittle behaviour at even the lowest temperatures.
Unlike the austenitic grades, the martensitic grades can be hardened by heat treatment. Certain martensitic grades contain nickel that improves the toughness but it also enables the steel to have a higher chromium content. This is once again to maintain a stable ferritic structure. This also increases the corrosion resistance of the grade.
Selecting a potential replacement grade for nickel-containing stainless steel
The selection of an alloy should be guided by careful examination of the application. Before making a change in an alloy, it is important to fully understand the current and potential alternatives in terms of the alloy’s strengths, weaknesses, and applicability to the application.
To counter poor choices, due to underestimating the required performance criteria, a solid understanding of both material and application is important. When considering grade selection, it’s important to remember that quality is in most cases more important than cost. Correct specification is key to delivering on the required levels of performance. Due to its longevity, it is always a good idea to calculate the cost over the complete life cycle costing (LCC). Stainless steel is surprisingly cost effective when not only the initial material cost is evaluated.
Using 200 series stainless steel
The 200 series of austenitic stainless steels have some nickel replaced by manganese. These stainless steels are regarded as an alternative to the 300 series. Both have an austenitic structure, with the 200 series containing manganese and nitrogen instead of some of the nickel. In practice, these materials work, look and feel like 300 series materials.
The original 200 series alloys were first produced in the US during the 1950s. The grades contained 16% chrome compared to the 18% in 304 and about 4% (half) the nickel content. Around 2005, a whole new range of 200 series alloys increased in popularity in Asia. To reduce the nickel content even further, producers had to reduce the chrome content even further - in some cases as low as 12%. As a result, the 200 series materials in general have a much lower corrosion resistance than their Series 300 counterparts. This does not reduce the applicability of the material where a slightly lower level of corrosion resistance is acceptable. In other cases, a higher rate of cold working is a benefit in applications such as hose clamps, some structural applications and reinforcing bar with a low magnetic response and a higher nickel content will be preferred.
Some 200 series alloys are also susceptible to delayed cold cracking after forming. This delayed cracking can in many cases only manifest months after the mechanical action. Welding of these grades can be more challenging than with the standard austenitic materials, especially in thicker sections. Keep in mind that the manganese addition might also influence the appearance of polished surfaces.
There are many legitimate applications for low chromium 200 series alloys, especially where there are lower demands on corrosion resistance. The selection of an alloy should be guided by a careful examination of all the requirements for the application.
Ferritic stainless steels with no nickel
In some applications, it might be possible to substitute an austenitic grade with a ferritic grade such as 430, 441 or 444. Grades 430 and 441 are nickel-free 17% chromium grades with good corrosion resistance and high-temperature strength. It has good formability and weldability making it a suitable replacement for 304 in indoor cladding, restaurant equipment and appliances, tubes, and heat exchangers.
Similarly, 316 can be substituted by 444. This is a nickel-free molybdenum-alloyed ferritic stainless steel with very good corrosion resistance, good cold formability, and high strength. It allows for thinner walls in tanks and is not prone to stress corrosion cracking. It is ideal for hot water tanks and drinking water pipes. Grade 444 shows a higher pitting resistance compared to 316.
Ferritic grades have good ductility and are therefore generally not suitable for load-bearing applications. They are also magnetic, unlike austenitic grades. In some uses, the difference in surface finish may be a significant factor as cold-rolled ferritic stainless steel has a slight shiny blue colouration, while austenitic grades appear grey. Ferritics do not harden or strengthen through cold work and would not be readily weldable above a 3mm gauge thickness.
Another characteristic of ferritic stainless steel is the high resistance to stress corrosion cracking, and they are usable in very aggressive conditions. The most common use of ferritic stainless steel is in the automotive industry for catalytic converters and exhaust systems. Ferritics display some mechanical properties, such as low-temperature toughness, generally poorer than austenitic grades.
Lean Duplexes looks like a long-term solution
Lean duplex grades are members of the duplex family. These alloys typically have a PRE number below 30, with a reduced content of expensive elements such as nickel and molybdenum. The grades aim to substitute 304 and 316 in more niche applications.
To maintain the desirable corrosion properties and phase balance between the ferritic and austenitic structures, other alloying elements are increased in the chemical composition, such as chromium, manganese, and nitrogen. Lean duplex is already quite popular for structural applications such as tanks, buildings, and bridges and the future will see increased usage in both the upstream and downstream oil and gas sectors, as well as in chemical, petrochemical, and food industries.
Advantages of using lean duplex
- The main reason for using lean duplex grades is its significant resistance to chloride stress corrosion cracking (SCC) because of its dual phases. As a rule of thumb, the corrosion resistance of lean duplex is likely to be similar to, or better than 304L and 316L with competitive PRE numbers.
- Unlike ferritic grades, the lean duplex grades are much more weldable in thicker sections.
- Lean duplex grades are not leaner on alloying nobility when compared to generally used austenitic grades such as 304 and 316.
- The yield strength of lean duplex grades is more than twice that of 304 and 316. This means that the wall thickness of equipment or piping can be reduced. Less thickness also means lower weight which can significantly lower initial material costs, transportation, and installation costs.
- Lean duplex benefits from an improved Life Cycle Costing (LCC) reducing the total cost and increasing the sustainability of the project.
Limitations of duplexes
However, there’s no such thing as a perfect material and hence some imitations when considering lean duplex:
- The duplex family achieve their phase balance and pitting corrosion resistance from nitrogen amongst others. During welding, some nitrogen is released from the metal, so the use of nitrogen-containing gas is recommended.
- The material, being less ductile and stronger than austenitics, requires more energy during forming and mechanical manipulation.
- The corrosion and mechanical properties of duplex grades are achieved with a ferrite/austenite phase balance of 40 – 60%. This is important to remember when selecting welding consumables, especially filler materials and weld input temperatures.
- Although the yield strength of lean duplex is more than double compared with austenitic, the allowable stress for design calculation is not.
- As in the case of 200 series austenitics, the manganese addition might also influence the appearance of polished surfaces.
Conclusion
Stainless steel is not one material, but a range of over 200 alloys, each with its unique properties and suitable for an endless variety of applications. Users and specifiers have for decades become accustomed to the versatility of the popular grades such as the nickel-containing 304s and 316s. They also have been spoilt by this and, in some ways, remained ignorant about the true range of stainless steel available. The correct material choice can have a significant impact on life cycle costs, profitability, and a sustainable future.