- Perspective – July 2022
- Advert : Columbus Stainless
- Industry Analysis
- Advert & Advertorial – EMV Africa
- Member Benefits
- Member Innovation
- Market Intelligence
- Advert – Beyond Stainless Steel Workshop
- Professional Profile
- Advertorial – Innov-X-Africa
- Advert – Innov-X-Africa
- Market Analysis
- Technical Analysis
- Technical Insight
- Advert – Unique Welding
- Africa Market Intelligence
- Member Events
WHAT IS ATMOSPHERIC CORROSION?
 Atmospheric corrosion is the deterioration and destruction of a material and its vital properties due to electrochemical reactions of its surface with the constituents of the atmosphere surrounding the material. Atmospheric corrosion does not discriminate, and it leads to the deterioration (corrosion) of metals and non-metallic materials. Environmental oxygen and condensed water vapour can cause gradual corrosion of iron and steel surfaces, producing iron oxide or rust. Corrosion drastically reduces the mechanical strength and useful life of the metals.
Atmospheric corrosion is the deterioration and destruction of a material and its vital properties due to electrochemical reactions of its surface with the constituents of the atmosphere surrounding the material. Atmospheric corrosion does not discriminate, and it leads to the deterioration (corrosion) of metals and non-metallic materials. Environmental oxygen and condensed water vapour can cause gradual corrosion of iron and steel surfaces, producing iron oxide or rust. Corrosion drastically reduces the mechanical strength and useful life of the metals.
The vital factor in atmospheric corrosion is the presence of moisture due to fog, dew, precipitation, and relative humidity. This provides the electrolyte for the ensuing electrochemical reaction. Salts of sulphur and chlorine can aggravate corrosion by increasing the conductivity of the water in industrial atmospheres. Ambient temperature and air pressure also affect corrosion. At higher temperatures, some electrolytes become highly reactive. The critical humidity which enables corrosion is a factor which is specific to each metal.
The study of atmospheric corrosion is essential because this type of damage is the most prevalent among the different types of corrosion damage.
This type of deterioration is widespread, as it affects outdoor as well as indoor installations such as utilities, industries, vehicles, and residential structures.
Factors that Affect Atmospheric Corrosion
Different factors are essential to atmospheric corrosion. Once these are identified within a specific environment they can contribute to the control of potential atmospheric corrosion.
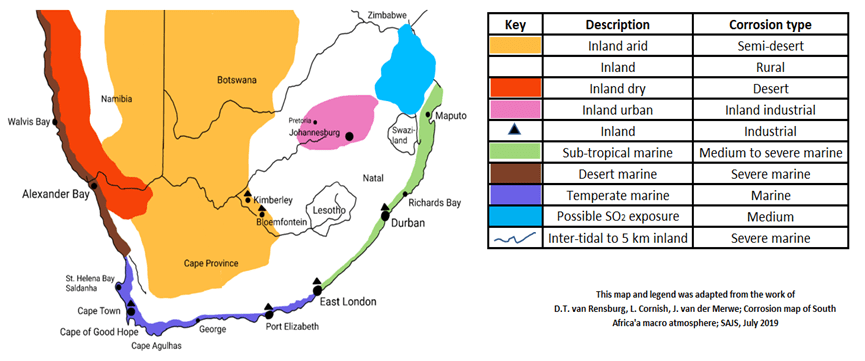
Figure 1 SA Corrosion map as adapted by Sassda
1.The environment:
The environment plays a critical role insofar as the risk for atmospheric corrosion is concerned. In complete rural environments, there will be little chemical pollution that is airborne to settle on material surfaces. In more urban areas the airborne pollution will increase due to emissions from the denser population, vehicles, and the like. This will increase as the environment changes from urban to industrial. It would therefore be important to identify the geographic location of the installation and potential sources of specific pollution in the area.
The South African corrosion map is used to determine corrosive conditions for the region. The map above was derived by Sassda from work done by the School of Chemical and Metallurgical Engineering, University of the Witwatersrand, South Africa. In essence, the effect of exposure to SO2 was added.
2.Proximity to the ocean and other sources of salt:
Halide ions such as chlorides are responsible for highly localised forms of corrosion such as pitting and crevice corrosion. In South African conditions, the proximity to the ocean plays a bigger role than in other parts of the world such as Europe. Heavy wave action and prevailing winds drive chloride-rich air deep inland. Other sources of salts will be mostly industrial or, as in the case in Europe, the use of de-icing salts on roads.
3.Climate:
Moisture, whether in the form of dew, rain or condensation, is a significant factor when it comes to atmospheric corrosion.
Although rain can wash away hazardous air pollutants in the atmosphere that have been deposited in exposed areas, such as in a marine environment, rain also collects in crevices and pockets. On such surfaces, moisture can become stagnant, which turns into an alkaline reaction with metal or absorbs carbon dioxide to create a dilute acid. Dew films that have become saturated with acid sulphates, sea salt and other acids can produce an aggressive electrolyte environment that promotes the occurrence of corrosion.
Temperature also affects atmospheric corrosion. As a rule of thumb, every 10°C increase in the temperature can double the corrosion rate.
The relative humidity is another important component in atmospheric corrosion. Among the requirements in the process of atmospheric corrosion is the existence of an electrolyte in the form of a thin film on metal surfaces that are exposed to critical humidity levels. Although the film is invisible, it can contain corrosive contaminants at high concentrations, particularly in situations where there is alternate drying and wetting.
4. Design factors:
While rain can wash down surfaces and, in effect, clean surfaces of contaminants that can lead to corrosion, flat or concave surfaces can lead to the collection of moisture to form puddles. This water can turn stagnant with a detrimental effect on the corrosion rate. This also applies to sheltered surfaces where no washing can take place. Examples of this would be overlapping surfaces, the undersides of horizontal sections, and applications where metal strapping is used. The surface finish of the material would also play a role. Rougher surfaces can trap contaminants while smooth surfaces will rid themselves of a potential build-up of pollutants.
5. Maintained schedule:
All surfaces need to be cleaned from time to time to rid the surfaces of contaminants that facilitate the creation of galvanic cells as part of atmospheric corrosion. High alloyed stainless steel might be corrosion resistant enough to withstand the corrosive attack, but maintenance and washing continue an important role in curbing atmospheric corrosion.
How to evaluate these factors in a standardised way?
The European standard EN 1993-1-4 describes a simple, yet effective methodology for doing this. The standard proposes that three risk factors be calculated; F1 the risk of exposure to chlorides; F2 the risk of exposure to sulphur dioxide; and F3 the risk in the cleaning regime. (Fig.2)
Figure 2 Selection of F1, F2, and F3
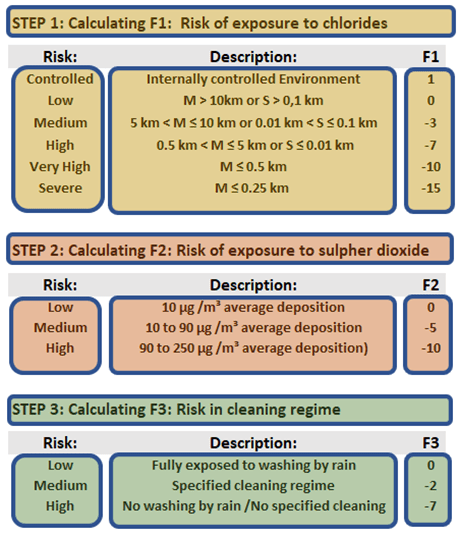
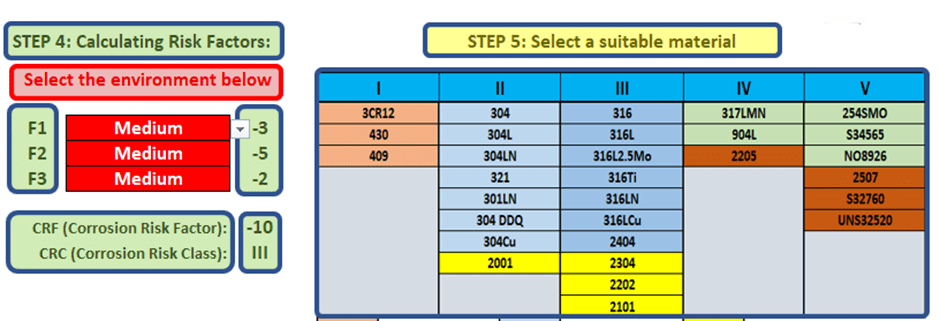
The total of the risk factors will then equal the Corrosion Risk Factor (CRF). The CRF is then used to determine a Corrosion Risk Class (CRC), and this is then used to determine the various grades that can be used in the specific application. (Fig.3)
Figure 3 Finding Risk Factor, Class, and material selection
It was also mentioned earlier that European conditions differ somewhat from that in Southern Africa. Especially in coastal areas, the African sub-continent sees much deeper penetration of chloride-rich air from the heavy wave action and strong prevailing winds. Fig.2 shows some adaptation to the values cited by the EN standard to compensate for the higher risk of chlorides in coastal areas.
For each risk class in fig.3, there is a variety of potential grades. It is advised to select one or two of these materials, and make use of sample plates, and to do a physical test in the real environment before the final decision on material grade is made.
Conclusion
Atmospheric corrosion is an underestimated form of corrosion and good initial material selection is often neglected. This leads to unnecessary damage, maintenance, and replacement costs. To play our part in a sustainable future, this unrelenting form of corrosion must be part of material selection and future sustainability. Atmospheric corrosion can be controlled and, in many cases, eradicated for all practical considerations.

