- Perspective – July 2023
- Best of the GPS Newsletter
- Webinar Report Back
- State of the Nation
- Multi Alloys & EMV Africa
- Demand Drivers – SA Rail
- Fastenright Advert
- Africa Profile
- Technical Insight
- Grinding Techniques Advertorial
- Technical Insight
- Technical Insight
- Pferd SA Advert
- Passionate Professional Profile
- Obituary – John Cluett
- Staff News
- PtSA Member Visit
- Steel Summit
- KZN Golf Day 2023
- Columbus Mill Visit
THE ‘ACID’ TEST WHEN IT COMES TO EFFECTIVE PASSIVATION
Post-fabrication treatment of stainless steel products, or damaged and contaminated surfaces remains critical to ensure that the material lives up to customer expectations. Stainless steel surfaces remain in pristine condition due to a very thin, tenacious chrome-oxide film that develops uniformly and continues when the material is exposed to oxygen in the environment. This film is stable and, most importantly, passive.
The passivity makes stainless steel surfaces inert and, as such, it does not readily react with the environment, even when in a corrosive environment. This property comes from the chrome content in the material since the more chrome stainless steel contains, the stronger the passivity and the corrosion resistance. The passive film is not a coating that needs to be maintained. It forms naturally and all it requires to remain strong, is unimpeded access to oxygen, simply meaning that the surfaces must be kept clean.
Pickle we must
 When stainless steel is produced at a mill and ready for dispatch, the passive film will be in optimum condition and pristine. However, along the process of the manufacturing value chain, a lot of things will happen to the material that will damage and impair the passive layer.
When stainless steel is produced at a mill and ready for dispatch, the passive film will be in optimum condition and pristine. However, along the process of the manufacturing value chain, a lot of things will happen to the material that will damage and impair the passive layer.
This includes multiple instances of transport and handling, exposure to ferrous and carbonaceous contamination; exposure to polluted atmospheres and the activities of fabrication such as cutting and forming. It is important to treat the surfaces after fabrication to ensure the removal of all embedded iron and metallic debris and dirt.
This will allow chrome to have free access to oxygen to build an effective passive film.
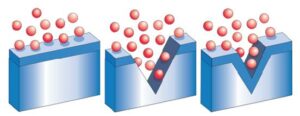
Some fabrication activities do not only impact the integrity of the passive film but also affect the material underneath the passive film and close to the surface. Heat treatment and welding are such examples. At high temperatures, heat oxide layers will form near the surface of the material. These layers are chrome depleted and cannot build and sustain a protective passive film. It needs to be removed to expose fresh material that can form a proper passive film.
Heat scale and most other surface defects can be removed by either mechanical methods or chemical methods. However, tests have indicated that chemical methods yield better results. It has been proven that the pickling process renders the most corrosion- resistant surface of all treatment methods.
Pickling normally refers to an acid mixture containing nitric acid and hydrofluoric acid that will remove the oxide scale and the underlying chromium-depleted layer. These acid mixtures are hazardous and must be handled with due care and disposed of correctly. As pickling would dissolve the stainless steel top surface, it must be carefully executed to the correct parameters.
Pickling can be performed by immersing the stainless steel part in a bath or by spraying the surface. Pickling products can also be applied locally in gel or paste forms. Irrespective of how pickling is conducted, it remains a necessary and critical step in surface restoration and ensuring the optimum performance of components and products in harsh environments.
To passivate or not to passivate?
The passive film forms naturally if there is unimpeded access to oxygen in clean and dry conditions. Pickling produces clean surfaces with a dull grey, matte finish that passivates spontaneously in the correct conditions. According to studies done by Outokumpu chemical passivation is rarely needed for improved corrosion resistance and is not required if the stainless steel has been properly pickled. On the other hand, passivation is an effective way to clean stainless steel that has not been pickled.
Chrome to the rescue
The answer to whether to passivate therefore lies in the conditions for natural passivation, the nature of the application, and the requirements of the end-user. The natural passivation process starts almost immediately once chemical contact is established between the environmental oxygen and the chrome contained in the material.
It is worth noting that the more chrome, the faster and stronger the passive layer will develop. It is also important to understand that all chemical processes on stainless steel such as cleaning, pickling and passivation, are governed by international standards.
The standard that the South African industry adheres to in terms of cleaning, descaling and passivation of stainless steel parts, equipment and systems is ASTM A380/A380M-13.
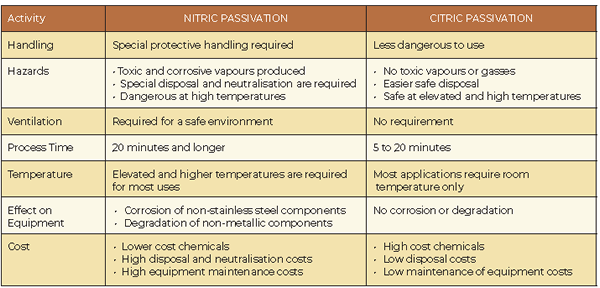
This standard allows for nitric acid based, as well as citric acid based methods for cleaning and passivation of stainless steel. The standards ASTM A967 and AMS 2700 states citric and nitric acid passivation to be effective for stainless steel parts.
Citric acid passivation
Citric acid passivation is the newer of the two processes and is also less used in the local industry. This technique was originally developed by the Coors Brewing Company to passivate the internal surfaces of beer kegs. Since citric acid is generally recognised as safe by the American FDA, it can be used safely in food and beverage applications.
Citric acid is the same non-toxic, biodegradable natural acid found in citrus fruits, making its use in passivation an environmentally friendly alternative to nitric acid. It also has fewer handling concerns than nitric acid. Unlike nitric acid, citric acid can be disposed of with minimal requirement of waste treatment.
Citric acid can passivate a wider variety of stainless steel alloys than nitric acid passivation. A citric acid passivation bath also takes far less time than nitric acid, speeding up the cleaning process considerably. However, for all these benefits, citric acid passivation is considerably more expensive, which is why many choose nitric acid.
According to ASTM A967, there are five different citric acid passivation methods:
- Citric 1: This solution has a strength of 4-10 w% citric acid, at a temperature of 60- 70 ̊C and 4 minutes minimum exposure time.
- Citric 2: This solution has a strength of 4-10 w% citric acid, at a temperature of 50- 60 ̊C and 10 minutes minimum exposure time.
- Citric 3: This solution has a strength of 4-10 w% citric acid, at a temperature of 20- 50 ̊C and 20 minutes minimum exposure time.
- Citric 4: This covers other combinations of temperature, time, and concentration of citric acid with or without chemicals to enhance cleaning, accelerants, or inhibitors capable of producing parts that pass the specified test requirements.
- Citric 5: This covers other combinations of temperature, time, and concentration of citric acid with or without chemicals to
enhance cleaning, accelerants, or inhibitors capable of producing parts that pass the specified test requirements, but with the immersion bath to be controlled at pH of 1.8 - 2.2.
Nitric acid passivation
Nitric acid passivation is the traditional method of passivation and has been used since the 1960s and is also the most used in the local industry. However, lower alloyed grades of stainless steel risk etching during the passivation process. This can be limited by adding sodium dichromate to the nitric acid, using a higher nitric acid concentration, or heating the nitric acid to a higher temperature.
Nitric acid chemistries with high oxidising potential are best, as the passive film formed on the surface forms faster and is more effective, thereby reducing the potential for etching. It has been stated that although nitric acid passivation is less costly to use, more pronounced environmental hazards exist. Nitric acid is naturally hazardous and emits toxic fumes. It also requires special handling and disposal, making it more expensive than using citric acid passivation in some situations.
According to ASTM A967 five different nitric acid passivation methods:
- Nitric 1: The solution consists of 20-25 v% nitric acid and 2.5 w% Sodium Dichromate, at a temperature of 45 -55 C with 20 minutes as minimum exposure time
- Nitric 2: The solution consists of 20-45 v% nitric acid, at a temperature of 20 -30 ̊C with 30 minutes as minimum exposure time.
- Nitric 3: The solution consists of 20-25 v% nitric acid, at a temperature of 50 -60 ̊C with 20 minutes as minimum exposure time.
- Nitric 4: The solution consists of 45-55 v% nitric acid, at a temperature of 45 -55 ̊C with 30 minutes as minimum exposure time.
- Nitric 5: This covers other combinations of temperature, time, and acid with or without accelerants, inhibitors, or proprietary solutions capable of producing parts that pass the specified test requirements.
From a purely environmental standpoint, citric acid passivation is a far better option. There are fewer handling concerns and less to worry about when it comes to disposal. But there are cases where nitric acid passivation works better. The risk of flash attack or the potential for etching the surfaces is elevated with nitric acid, however, taking the precautions mentioned earlier lessens the risk. Flash attack can occur with citric acid too, but at a much lower risk.
Early citric acid formulations suffered from organic growth issues, but modern formulations include biocides to mitigate the issue. Citric acid removes only the free iron on the surface, whereas nitric acid removes some metals on the alloy itself. Citric acid is more expensive since it is naturally derived and can’t be created in a laboratory. This results in a higher chemistry costs, even as it saves money in just about every other area, including labour, equipment, maintenance and disposal costs.