- Perspective – November 2022
- Columbus Advert
- Market Intelligence
- Unique Welding Advert
- Industry Analysis
- Industry Insight
- Market Intelligence
- Industry Networks
- Advertorial – NDE
- Webinar Report Back
- Schultz Incorporated Advert
- Case Study
- Professional Profile
- Inox Systems Advert
- Africa Focus
- Member Insight
- Training Focus
- Obituary – Vaughan Davis
- Member Focus
HOW TO MAINTAIN & PROTECT STAINLESS STEEL’S MAGICAL PASSIVE LAYER
Sassda has hosted a range of 60Minutes with Stainless webinars this year with great success. One of the most popular of these focused on stainless steel’s almost magical and highly protective passive layer that gives it the ability to ‘fight’ off a range of threats to the superior corrosion resistance of the material. However, the passive layer is constantly bombarded by external factors and it also comes under acute pressure during fabrication when the integrity of the passive layer is pushed to the max. Read on to gain a full overview of the information discussed during the webinar and discover why and when chemical restoration is important for the passive layer…
A stainless steel surface should appear clean, smooth and faultless. This is obvious when the steel is used for such purposes as façades or in applications with stringent hygienic requirements, but a fine surface finish is also crucial to corrosion resistance.
Stainless steel is protected from corrosion by a thin, impervious, invisible surface layer - the passive layer - that consists mainly of chromium oxide. The oxygen content of the atmosphere or aerated aqueous solutions is normally sufficient to create and maintain this passive layer.
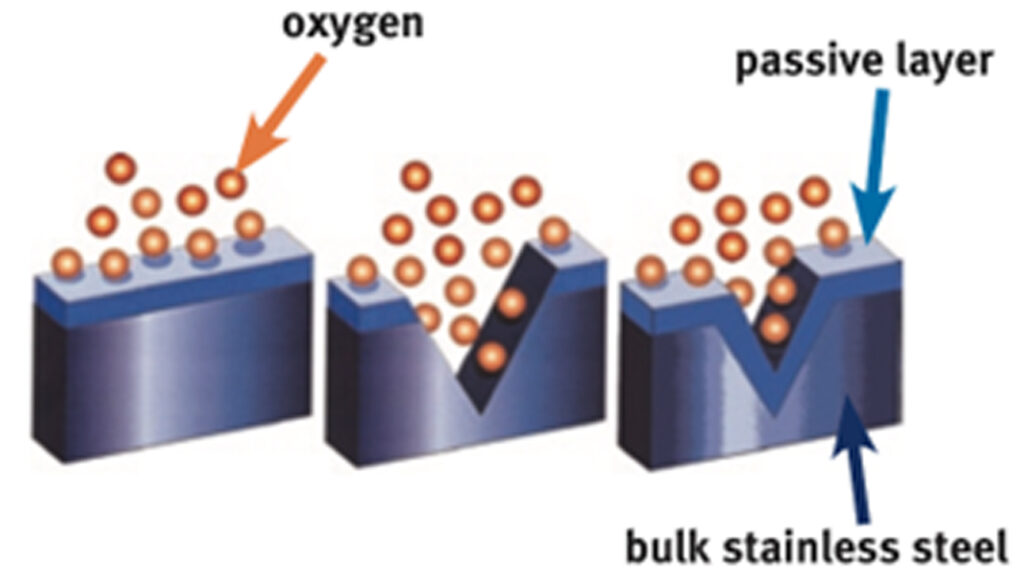
Unfortunately, surface defects and imperfections introduced during manufacturing operations may drastically disturb this ‘self-healing’ process and reduce resistance to several types of local corrosion. This means that a final cleaning process will often be required to restore an acceptable surface for hygiene and corrosion considerations be determined by the corrosivity of the environment, the corrosion resistance of the steel grade, hygienic requirements (e.g., in the pharmaceutical and food industries), or purely aesthetic considerations.
Consideration must also be paid to local environmental requirements. Both chemical and mechanical cleaning methods are available. Good design, planning and methods of manufacture can reduce the need for finishing work and thus reduce costs. The influence of defects and ultimately their removal must be considered when manufacturing to specifications that relate to certain surface quality requirements.
The typical defects that will be removed by mechanical or chemical treatment would include:
- Heat tint and oxide scale -High temperature oxidation caused by processes such as heat treatment or welding produces an oxide layer with inferior protective properties, compared with those of the original passive layer. A corresponding chromium depletion in the metal, immediately below the oxide also occurs. The chromium-depleted zone under normal welding heat tint is very thin and can normally be removed together with the tint. It is, however, necessary to remove this layer to completely restore corrosion resistance.
- Weld defects - Incomplete penetration, undercut, pores, slag inclusions, weld spatter and arc strikes are typical examples of weld defects. These defects have negative effects on mechanical properties and resistance to local corrosion and make it difficult to maintain a clean surface. The defects must therefore be removed, normally by grinding, although sometimes repair welding is also necessary.
- Iron contamination - Iron particles can originate from machining, cold forming and cutting tools, blasting grits/sand, or grinding discs contaminated with lower alloyed material, transport or handling in mixed manufacture or simply from iron-containing dust. These particles corrode in humid air and damage the passive layer. Larger particles may also cause crevices. Reduced corrosion resistance will result in both cases. This type of corrosion produces unsightly discolouration and may also contaminate the media used in the equipment in question.
- Organic contamination - Organic contaminants in the form of grease, oil, paint, footprints, glue residues and dirt can cause crevice corrosion in aggressive environments. This renders surface pickling activities ineffective and pollutes products handled in the equipment. Organic contaminants should be removed using a suitable pre-cleaning/degreasing agent (chlorine-free). In simple cases, a high-pressure water jet can be used.
Different chemical and mechanical methods, and in certain instances a combination of both, can be used to remove the defects mentioned. Generally, cleaning based on chemical methods can be expected to produce superior results since most effective mechanical methods tend to produce a rougher surface while chemical cleaning methods reduce the risk of surface contamination. However, local regulations relating to environmental and industrial safety as well as waste disposal problems may limit their application.
Mechanical methods will include:
- Grinding - Grinding is normally the only method that can be used to remove defects and deep scratches. A grinding disc is usually adequate for treating defects of this type. The grinding methods used should never be rougher than necessary and a flapper wheel is often sufficient for removing weld tint or surface contamination.
- Blasting - Sand and grit blasting (peening) can be used to remove high temperature oxide as well as iron contamination. However, care must be taken to ensure that the sand (preferably of olivine type) or grit is perfectly clean. The blasting material must therefore not have been previously used for carbon steel, nor should the sand or grit be too old, as it becomes increasingly polluted, even if it is only used for blasting contaminated stainless steel surfaces. Surface roughness is the limiting factor for these methods.
- Brushing - For the removal of heat tint, brushing using stainless steel or nylon brushes usually provides a satisfactory result. These methods do not cause any serious roughening of the surface, but also don’t guarantee the complete removal of the chromium-depleted zone. With other mechanical methods, the risk of contamination is high and it is therefore important that clean tools that have not been used for processing carbon steels are used.
Chemical methods can remove high temperature oxide and iron contamination without damaging the surface finish. Electropolishing may also improve the surface finish. Since they remove the surface layer by controlled corrosion, chemicals will also selectively remove the least corrosion-resistant areas such as the chromium-depleted zones.
Chemical methods would include:
- Electropolishing - Electropolishing normally produces a surface that guarantees optimal corrosion resistance. The material gains a fine lustre, and, above all an even microprofile that meets extremely stringent hygienic requirements.
- Pickling - Pickling is the most common chemical procedure used to remove oxides and iron contamination. Thorough rinsing with clean tap water must follow pickling. A final pickling/cleaning operation following a typical manufacturing programme may include:
- Grinding for removal of defects caused by welding as slag must be removed after welding
- Organic contaminants should be removed using a suitable precleaning/ degreasing agent (chlorine-free). In simple cases,
a high-pressure water jet can be used
- Pickling using a bath, paste or solution, possibly in combination with a careful mechanical treatment to break oxides.
- A thorough rinsing with water, preferably using a high-pressure water jet.
- Passivation and decontamination - Procedure is similar to pickling, but in this case, the active agent is nitric acid only. The acid is applied by immersion or spraying. This treatment strengthens the passive layer. The treatment is more important after mechanical cleaning and operations involving a risk of iron contamination since the acid also removes iron impurities from the surface.
This method could also be referred to as decontamination given that after every acid treatment, rinsing with water is vital. After certain types of production processes, passivation/decontamination may suffice as a cleaning method. This method is also strongly recommended after mechanical treatment as well as after pickling in special cases.
The choice of method and the extent of final cleaning required will depend on the need for corrosion resistance, hygienic considerations (pharmaceuticals, food) or whether visual appearance is the sole criterion.
The routine removal of welding defects, welding oxides, organic substances and iron contaminants is normally a basic requirement and usually allows a comparatively free choice of final treatment. Provided that the surface roughness permits, mechanical and chemical methods can be used.
However, if an entirely mechanical cleaning method is considered, the manufacturing stage has to be well planned. This is to avoid iron contamination, since decontamination, probably with nitric acid, will otherwise be necessary. When requirements for surface finish and corrosion resistance are exacting, the choice of method is more critical. A treatment sequence based on pickling will in such cases provide the best chances of a superior result.

