3CR12 is a Chromium-Containing Corrosion-Resistant steel that conforms to a number of international standards. It has proven useful in applications where the corrosion resistance of carbon steel or even galvanized steel is considered to be insufficient. Since its introduction in 1979, 3CR12 has been used extensively in aqueous environments. Its resistance to general corrosion has often been good, but as with any material relying on the formation of a passive film for corrosion resistance; its localized corrosion resistance depends upon many factors. It is important to note these 5 factors to be considered when selecting 3CR12 for aqueous service.
1) Design
In this context, design refers to maximizing the corrosion performance. The following design guidelines apply.
- 3CR12 is noble to most other materials. A non-porous insulator should be used between 3CR12 and other materials. Consideration can also be given to coating the joint area to avoid galvanic attack.
- Crevices should be designed out of the system. Water retention, which would permit the build-up of silt and salts, must be avoided. Angles therefore should point downward, and not upward.
- Free drainage must be provided for. Where possible, corners should be avoided at the bottom of tanks. Piping should be sized to ensure adequate flow to prevent suspended solids from settling out.
- 3CR12 may discolour in aqueous media. However, there is usually no contamination of the water or loss of material thickness.
- Design should permit access to the structure for initial fabrication and possible repair to the required standard when using 3CR12.
- Avoid stagnant water conditions. Changes to the environment such as aeration, settling of solids, may result in conditions that could be detrimental to the material.
- Closed 3CR12 water conveyance systems should be coated. Epoxy coated 3CR12 outperforms epoxy coated carbon steel piping even in aggressive conditions. Holidays in coating systems would cause corrosion of the substrate steel. 3CR12 is more forgiving as the extent of corrosion product formed does not cause blistering of the coating as is the case with carbon steel. The choice between coated and uncoated 3CR12 should be based on a thorough water quality analysis, including microbial count.

The Municipal water tank (right) manufactured from 3CR12 instead of concrete; the manufacturer won an award from SASSDA in 2018. |
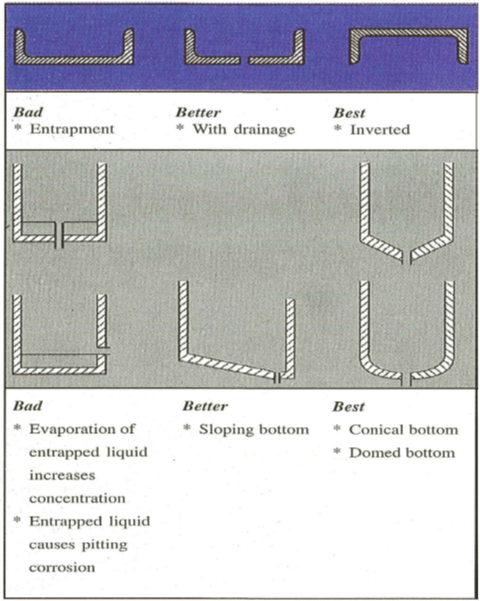
Figures 1(a-b): Design considerations |
2) Fabrication
3CR12 should be handled in the same way as any other stainless steel grade, with every effort being made to ensure cleanliness both in the workshop and on-site.
- Mild steel and carbonaceous contamination can cause significant discolouration and potential loss of corrosion resistance and thus should be avoided.
- 3CR12 can be formed, machined, and welded by commonly used industrial process. Poor fit-up must be avoided, and competent welding practices should be followed to ensure smooth joints.
- Post-fabrication cleaning and subsequent chemical passivation is essential for 3CR12 when it is to be used in aqueous environments. All welding processes give rise to a zone of discolouration in the weld area. This can provide a site for corrosion, and must be removed.
3) Aeration and flow rates
Aeration is beneficial to the overall performance of stainless steel, including 3CR12. The presence of oxygen is essential to the formation and maintenance of the protective passive film. Flow rates should therefore be sufficient to maintain high oxygen levels in the aqueous stream. Stagnant conditions should be avoided, as localized attack of the material may then occur.
4) Suspended Solids, Scaling and Fouling
- Suspended solids: Sufficient flow should be maintained in order to prevent any suspended solids from settling out. Should settling occur, localized corrosion may well result. Moving mineral solids should not present a corrosion risk
- Fouling: Microbial induced corrosion (MIC) is a problem which could be considered specific to each and every application. In sewerage applications (weirs, baffle plates, etc.) 3CR12 has performed admirably in the presence of high bacterial counts. Bacteria in this application have shown not to result in microbial induced corrosion, provided that observable bio fouling is minimized.
5) Water Quality and Temperature
- pH value between 3 and 9 has no apparent effect on the pitting performance in deaerated environments.
- Alkalinity: Increasing alkalinity inhibits the initiation and propagation of pitting. However, very high alkalinity may lead to the precipitation of an insoluble carbonate, which may promote under-deposit attack on the metal surface.
- Microbial activity: Sulphate reducing bacteria (SRB) and iron oxidising bacteria have been cited as the most common causes of MIC in 3CR12. SRB associated corrosion occurs in anaerobic conditions in the presence of water, and is affected by flow rates - beyond a certain flow rate bio fouling ceases. The mode of attack is pitting rather than general corrosion.
- Chloride: Chloride ions have a detrimental effect on the localized corrosion of stainless steels by enhancing the localized dissolution of the thin passive layer. Increasing chloride ion concentration and temperature reduce the localized corrosion resistance of the material. Critical pitting temperature diagrams (CPT) are often produced in order to ascertain the propensity of the material to pitting attack. These diagrams are produced for waters containing chlorides only. The CPT diagram for 3CR12 is shown (Figure 2a). In waters where other ionic species are present, caution should be exercised in taking a particular numerical value for pitting potential.
- Sulphate and Nitrate: Both sulphate and nitrate ions have a marked inhibiting effect on localized corrosion. Nitrate is a more efficient inhibitor than sulphate. For a given content of inhibiting anions, there is a chloride level, defined as the critical chloride concentration, below which pitting is completely inhibited. A linear relationship exists between the critical chloride concentration and sulphate and nitrate, both in isolation and in combination, as indicated in the model below, Figure 2b. (Note: A straight line drawn between the concentrations of sulphate and nitrate will intersect the chloride axis at the maximum permitted chloride concentration for this water).
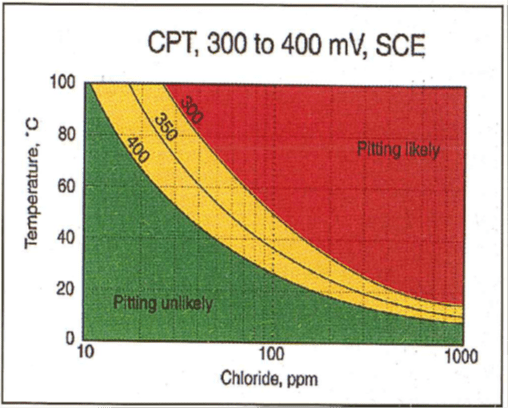
Figure 2(a-b): CPT diagram for 3CR12 |
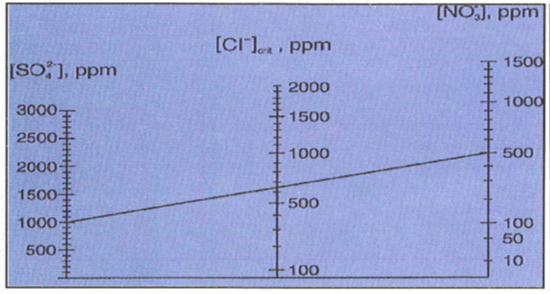
The Model for predicting the maximum Cl- ion content from known sulphate and nitrate contents |
For any further information please email Lerato Mashigo - mashigo.lerato@columbus.co.za

