- About Stainless
- 3CR12
- Care, Maintenance and Cleaning of Stainless Steel
- Colour Coding Chart
- Facts About Stainless
- Frequently Asked Questions
- World Stainless Association
- Introduction to Stainless Steel
- Life Cycle Costing and Stainless Steel
- LCC Software
- Maintaining Stainless Steel in and Around the House
- Stainless Steel In Architecture
- Preamble for Architects
- Standard A380/A30 M for cleaning and passivation of stainless steel
- Stainless Steel and the Environment
- Stainless Steel Roofing
- Surface Finishes
- Technical Advice & Support
- Technical Enquiries
- Types of Stainless
- Typical Applications
Stainless steel represents one of the more recent groups of engineering materials. Although invented at the beginning of the 20th Century, it took several decades before their use became widespread. It was not until after the World War 2, that modern stainless steels were developed and commonly used.
The single most important property of stainless steel is their corrosion resistance. Corrosion resistance, in combination with good mechanical properties and manufacturing characteristics, has helped establish stainless steel as an extremely versatile material which, in many cases, offers the only economically viable alternative for the designer.
What makes stainless steel stainless ?
Stainless steel is an alloy of iron (FE) and chromium (Cr), with a controlled amount of carbon (C). They are a family of steels containing a minimum of 11% chromium, whose primary property is that of corrosion resistance. If 11% of more chromium is added, a protective, passive film will form. The higher the chromium content, the stronger the passive film.
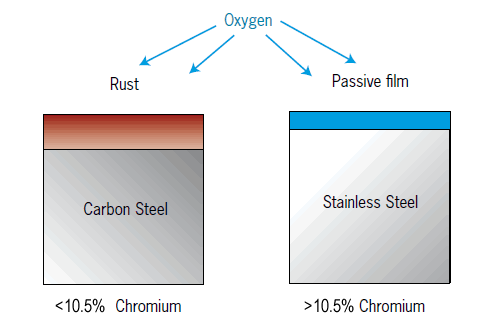
Other elements such as molybdenum (MO) and Nitrogen (N) further strengthen the passive film and improve corrosion resistance. If the passive film is removed or damaged, it will spontaneously re-form in the presence of air or water.
Why then is Nickel added ?
Nickel makes stainless steels austenitic, and counts for the austenitic grades excellent properties, namely weldability, formability and toughness etc.
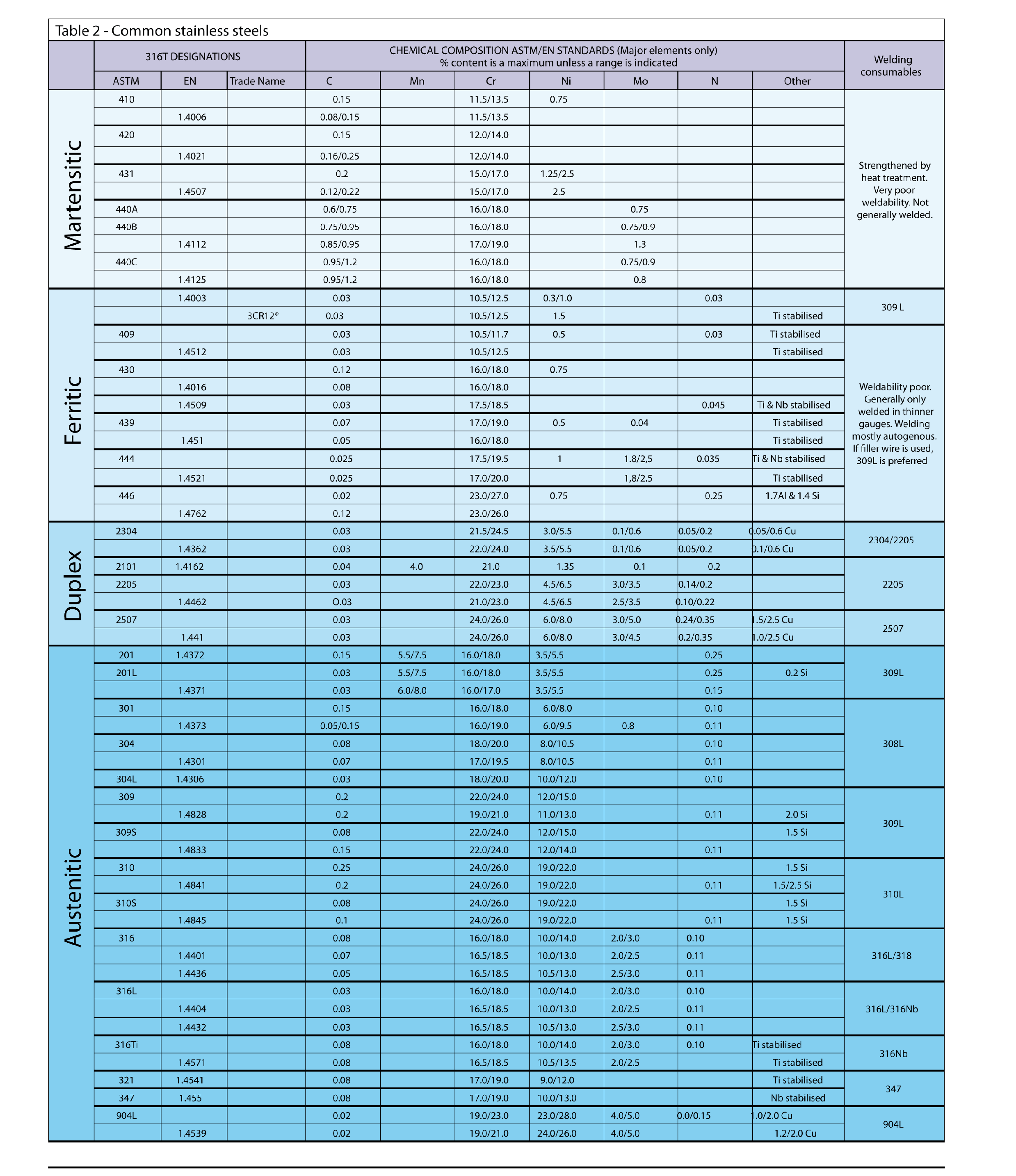
Stainless steel classification and grades
Since their invention and initial development, the number of stainless steel grades has increased rapidly, with hundreds of different chemical compositions standrdised around the world.
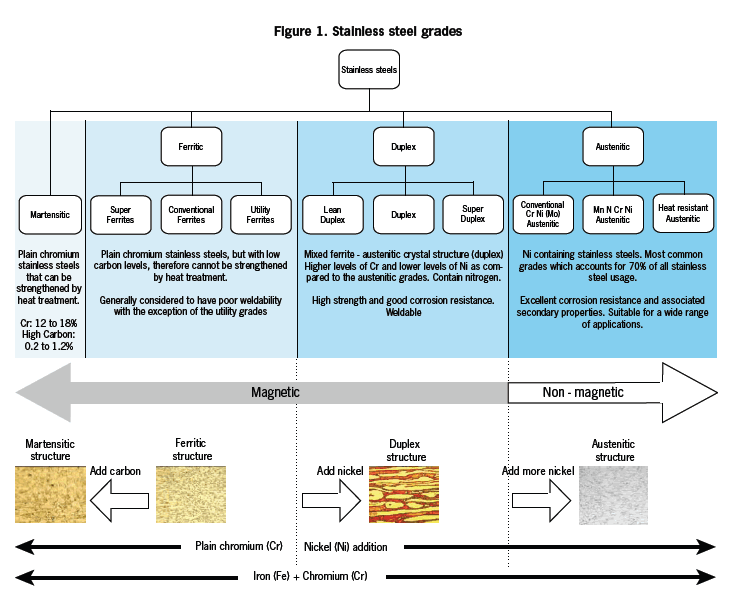
Since the micro structure of the steel determines its properties, stainless steel have traditionally been divided into categories based on their structures. This gives a rough classification in terms of both composition and properties. The relationship between the various stainless steel classifications is summarised below and is schematically illustraded in figure .
Martenstic : Are plain chromium alloys with relatively high carbon levels. Are included in the American Iron and Steel Institute (AISI) 400 series. These steels have a moderate corrosion resistance, high strength and hardness developed by heat treatment. They have poor weldability and are magnetic.
Ferritic : Are plain chromium alloys with low carbon levels and are also included in the AISI series. These steel have moderate to excellent corrosion resistance depending on chromium content. They cannot be strengthened or hardened and have poor weldability except in thin gauges. They are magnetic.
Austenitic : These steels are included in both the AISI 300 and AISI 200 series and contain nickel with low to very low carbon contents. These materials have excellent corrosion and high temperature oxidation resistance. Strength and hardness can be increased by cold work. They have excellent cryogenic properties. They are non-magnetic. In steels in the 200 series, the nickel is partially replaced by manganese. These variants have properties similar to the 300 series, but have higher strength and lower corrosion resistance.
Duplex : These steels contain less nickel than the austenitic alloys and have very low carbon contents. They have a duplex (mixed) crystal structure of ferrite and austenite. These steels have excellent corrosion resistance, particularly to pitting, crevice corrosion and stress corrosion cracking. They also have high strength, excellent weldability and are magnetic.
Corrosion Resistance
Corrosion resistance of stainless steels increases with increasing levels of chromium and additions of molybdenum and nitrogen. The relative corrosion performance of selected stainless steels under atmospheric conditions is illustrated below